Oxidative Phosphorylation & Electron Transport Chain Explained: Steps, Complexes & ATP Formation
Nov 20, 2025
Electron Transport Chain
All complexes of ETC are present along the Inner side of the inner mitochondrial membrane.
Oxidative Phosphorylation
The Electron Transport Chain (ETC) is located on the inner side of the inner mitochondrial membrane.
Oxidative phosphorylation is a part of catabolism, where biomolecules break down into simpler substances, releasing energy. This released energy is not directly stored as ATP but first trapped as reducing equivalents — NADH and FADH₂.
When NADH and FADH₂ are oxidized, they release electrons that pass through the ETC, producing energy. This energy is then used to phosphorylate ADP into ATP, which is the main purpose of oxidative phosphorylation. Hence, the ETC acts as the main tool for oxidative phosphorylation.
Only NADH and FADH₂ can donate electrons to the ETC, not NADPH, as NADPH does not produce energy this way.
Glycolysis (in the cytoplasm) produces 2 NADH per glucose molecule, but since NADH cannot cross the mitochondrial membrane, special shuttles are used to transport its electrons into the mitochondria, the Malate shuttle and the Glycerol phosphate shuttle.

Malate Shuttle
In the malate shuttle, NADH is transported in the form of malate. For this purpose, two enzymes will be used- cytoplasmic malate dehydrogenase and mitochondrial malate dehydrogenase.
Cytoplasmic NADH is converted into malate by cytoplasmic malate dehydrogenase, which uses oxaloacetate as a substrate. Malate then crosses the mitochondrial membrane, where mitochondrial malate dehydrogenase converts it back to oxaloacetate, regenerating NADH inside the mitochondria.
This allows the transfer of reducing equivalents from the cytoplasm to the mitochondria for ATP production. The malate shuttle functions in most cells except white muscle fibers and neurons, which use the glycerol phosphate shuttle instead.
|
|
Glycerol Phosphate Shuttle
In the glycerol phosphate shuttle, NADH is transported in the form of glycerol. For this purpose, two enzymes will be used- cytoplasmic glycerol-3-phosphate dehydrogenase and mitochondrial glycerol-3-phosphate dehydrogenase.
Cytoplasmic glycerol-3-phosphate dehydrogenase transfers electrons from NADH to dihydroxyacetone phosphate (DHAP), forming glycerol-3-phosphate. This molecule carries the electrons into the mitochondria, where mitochondrial glycerol-3-phosphate dehydrogenase converts it back to DHAP, transferring the electrons to FAD to form FADH₂. The resulting FADH₂ then enters the electron transport chain to help generate ATP.
This glycerol phosphate shuttle mainly operates in white muscle fibers and neurons.
Difference between the malate shuttle and the glycerol phosphate shuttle
The main difference between the malate and glycerol phosphate shuttles is in the form of reducing equivalents produced inside the mitochondria. In the malate shuttle, cytoplasmic NADH becomes mitochondrial NADH (yielding 2.5 ATP), while in the glycerol phosphate shuttle, cytoplasmic NADH becomes mitochondrial FADH₂ (yielding 1.5 ATP).
This causes a loss of 1 ATP per NADH. Since glycolysis produces 2 NADH per glucose, using the glycerol phosphate shuttle results in a total loss of 2 ATP per glucose.

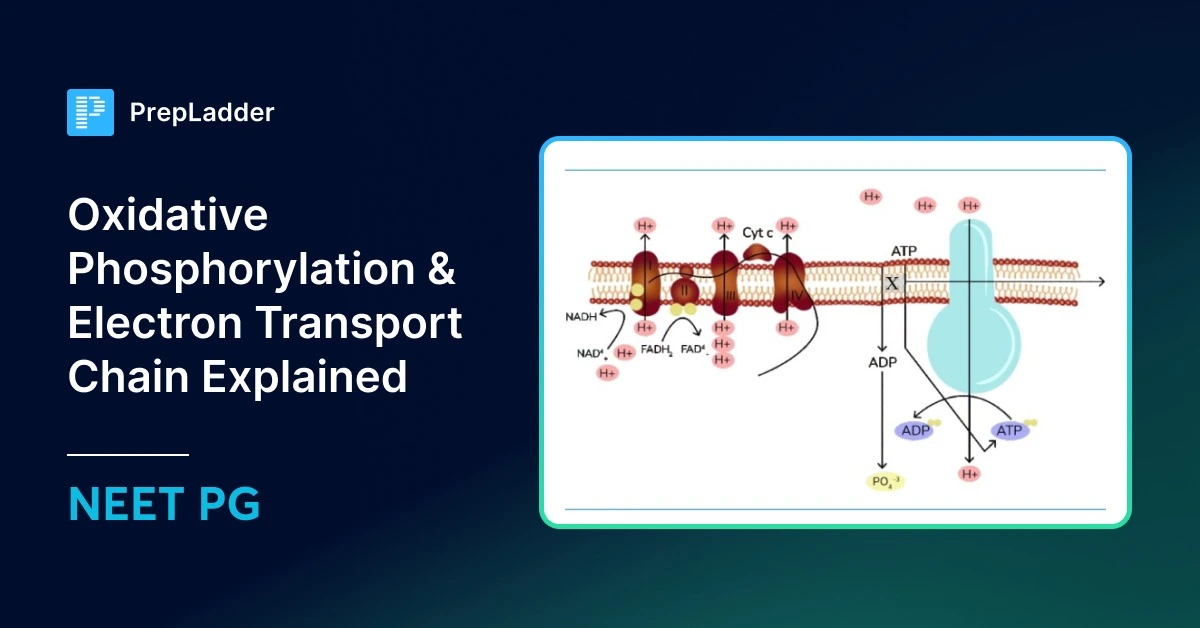
Complexes of the electron transport chain
All electron transport chain complexes are made up of cytochrome heme proteins, except coenzyme Q (complex Q). Heme proteins have a heme group with a central metal atom (usually iron) surrounded by four pyrrole rings.
In non-cytochrome heme proteins like hemoglobin and myoglobin, the central Fe²⁺ cannot change its oxidation state; if it becomes Fe³⁺, it forms methemoglobin and cannot bind oxygen.
In cytochrome heme proteins, the central metal can switch between oxidation states (Fe²⁺ ↔ Fe³⁺), allowing electron transfer in the ETC. For example, in Complex I, Fe³⁺ becomes Fe²⁺ when it accepts electrons from NADH and returns to Fe³⁺ when it donates them.
All ETC complexes contain iron, except Complex IV (cytochrome a and a₃), which contains copper (Cu) at its center.
There are 5 stationary complexes and 2 mobile complexes:
5 stationary complexes
- Complex I: NADH dehydrogenase — receives electrons from NADH.
- Complex II: Succinate dehydrogenase — receives electrons from FADH₂.
- Complex III: Contains cytochromes b and c₁.
- Complex IV: Contains cytochromes a and a₃.
- Complex V: ATP synthase complex, makes ATP using the proton gradient.
2 mobile complexes:
- Coenzyme Q (ubiquinone) – between Complex I/II and III.
- Cytochrome C – between Complex III and IV.
Electrons from NADH enter the electron transport chain at Complex I, then move to Coenzyme Q, Complex III, Cytochrome C, and finally to Complex IV, where oxygen (O₂) receives them as the final electron acceptor.
Electrons from FADH₂ start at Complex II and follow the same route: Coenzyme Q → Complex III → Cytochrome C → Complex IV → O₂.
Besides succinate dehydrogenase, other FADH₂-linked enzymes like acyl-CoA dehydrogenase (from fat breakdown) and mitochondrial glycerol-3-phosphate dehydrogenase also send their electrons directly to Coenzyme Q and follow the same pathway.
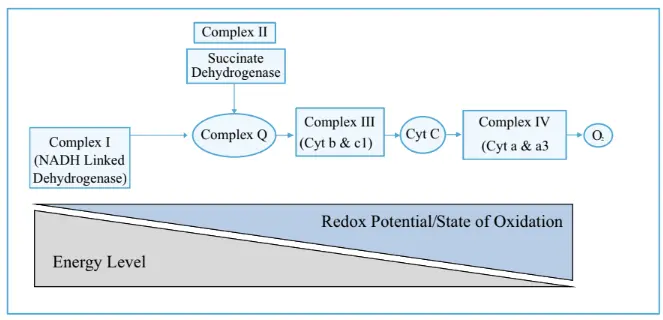
Production of ATP from ETC complexes was given by Mitchell's chemiosmotic theory. According to this theory, ATP is produced as electrons pass through ETC complexes arranged in increasing redox potential (decreasing energy levels).
More reduced substances have higher energy, and as they get oxidized, energy is released. This energy is used to pump hydrogen ions (H⁺) from the mitochondrial matrix to the space outside the inner mitochondrial membrane, creating a proton gradient.

All the ETC complexes are located on the inner side of the inner mitochondrial membrane (the green ring). As electrons move through the complexes, hydrogen ions (H⁺) are pumped from the matrix to the space outside the inner membrane, creating a proton gradient. These H⁺ ions then pass back into the matrix through the Fo component of ATP synthase (Complex V).
Complex V has two parts: Fo, which forms the ion channel for H⁺ movement, and F₁, which is the actual ATP-synthesizing enzyme. When hydrogen ions flow from a region of high to low concentration through the Fo channel, energy is released, and this energy is used by the F₁ unit to phosphorylate ADP into ATP. This is how the electron transport chain drives the formation of ATP.
Q. Why is 2.5 ATP for NADH and 1.5 for FADH2? Why does this discrepancy occur?
Ans. It is because NADH enters ETC through Complex I, and there is an energy difference between Complex I and Q. When an electron moves from Complex I to Complex Q, 4 hydrogen atoms can be translocated. The number of electrons that can move depends on the energy that is liberated.
The energy that is liberated will depend upon the energy difference. The energy difference between the complex I and Q is such that when electrons move from I to Q, 4 hydrogen ions get translocated. And the energy difference between the complex Q and III is such that when electrons move from Q to III, again 4 hydrogen ions get translocated.
But the energy difference between the complex III and IV is such that when electrons move from III to IV, only 2 hydrogen ions get translocated. A total of 10 hydrogen ions are translocated when the NADH electron enters the ETC chain.
ATP synthase complex works in such a way that when 4 hydrogen ions go through the Fo component, 1 ATP is generated. 4 ions help in generating 1 ATP, and 10 ions help in generating 2.5 ATP. That is why it is 2.5 ATP for NADH.
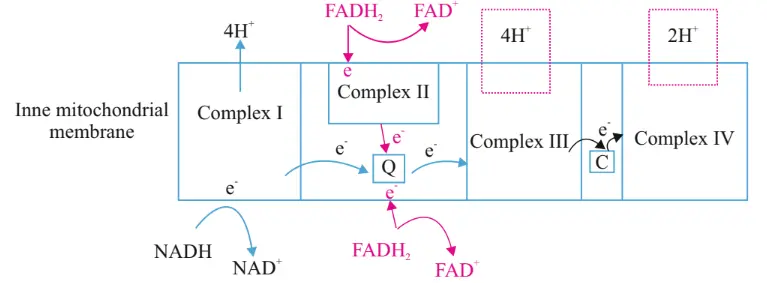
FADH2 enters ETC either through complex II or directly get into through complex Q. In either case, Complex II and Q have the same energy level. Because they have the same energy level, no hydrogen atom will translocate. From Q to III, 4 hydrogen ions will translocate. From III to C, 2 hydrogen ions will translocate.
Totally 6 hydrogen ions are translocated when the FADH2 electron enters the ETC chain. 4 ions help in generating 1 ATP, and 6 ions help in generating 1.5 ATP. That is why it is 1.5 ATP for FADH2.
Inhibitors of ETC
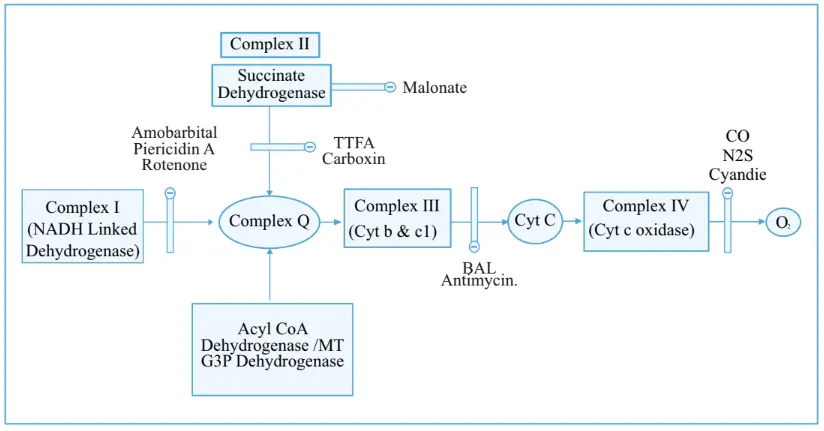
The electron transport between complex I and complex Q is inhibited by amobarbital, piericidin A, and retinol. Complex II or Q is inhibited by malonate. The electron transport between complex II and complex Q is inhibited by TTFA carboxin. The electron transport between complex III and complex C is inhibited by BAL antimycin.
The electron transport between complex IV and oxygen is inhibited by hydrogen sulfide, cyanide, and carbon monoxide. Cyanide causes histotoxic hypoxia does not matter how much oxygen is available, tissues will not be able to extract oxygen because electron transport is inhibited by cyanide. The major mechanism by which carbon monoxide exhibits toxicity is that carbon monoxide exhibits a very high affinity to the Fe2+ of hemoglobin, so it displaces oxygen.
The oxygen-carrying capacity of hemoglobin reduces, and this is called anemia. So carbon monoxide causes anemic hypoxia. Any electron transport inhibitor will cause histotoxic hypoxia.
Uncoupler
The major function of ETC is that it couples the oxidation of fuel with the phosphorylation of ADP to form ATP. The uncoupler uncouples oxidation from phosphorylation. It means oxidation of fuel will be done continuously without phosphorylation.
Effects of uncouples
- The first effect of the uncoupler will be that there will be no ATP production.
- Increase in the rate of heat production.
- An increase in the rate of oxidation of all the fuels will increase the basal metabolic rate.
Definition of uncouplers
All uncouplers are ion channels, and they get inserted into the inner mitochondrial membrane. Through these ion channels, hydrogen ions will bypass. Because hydrogen ions do not go through Fo components, ATP will not be produced.
Here also hydrogen ions also move from regions of higher concentration to regions of lower concentration, so the energy will be liberated as heat.
Types of Uncouplers
Two types of uncouplers are physiological uncouplers and artificial uncouplers.
Physiological uncouplers
They include thyroxine and brown adipose tissue. Thyroxine causes upregulation of uncoupler proteins in the inner mitochondrial membrane through which hydrogen ions pass; no ATP, no heat production occurs, which is why thyrotoxicosis presents with heat intolerance, and that is why hypothyroidism presents with cold intolerance.
For want of ATP, more and more fuels will be oxidized in the presence of thyroxine. Brown adipose tissue is of brown colour because it has numerous mitochondria, and in all these inner mitochondrial membranes, uncoupler proteins are inserted. Through these uncouplers, hydrogen ions bypass, which is why no ATP production and only heat production will occur.
Brown adipose tissue helps in heat production and they are considered a mechanism for non-shivering thermogenesis in neonates and hibernating anime. Neonates do not exhibit shivering, but they have to generate heat. At the time of birth, we are all born with an adequate amount of brown adipose tissue.
For example, the interscapular region has lots of brown adipose tissue in the neonatal period that helps in heat production. As age progresses, the brown adipose tissue regrases. In some people, this brown adipose tissue is retained, and that is actually quite fortunate.
That means any fuel they will intake will be oxidized, and energy will be liberated as heat. It doesn't couple to ATP production, and there will be no anabolism and weight gain.
Artificial Uncouplers
Artificial uncouplers include 2,4 2,4-dinitrophenol, valinomycin, neomycin, and harrison. These 4 were once tried as anti obesity drugs. In the presence of these uncouplers, all the fuels will be oxidized and energy will be liberated as heat, with no ATP production, and no anabolism.
Most of them are presented as increased heat production, and many of them are posterior subcapsular cataracts; they all withdrew from the market.
Oligomycin
Oligomycin acts by inhibiting the Fo component of the ATP synthase complex. It is denoted by Fo to show that it is inhibited by oligomycin. In the presence of oligomycin, hydrogen atoms will not enter mitochondria through the ATP synthase complex. So there will be no ATP synthesis.
The difference between this and other uncouplers is that in other uncouplers, hydrogen ions somehow manage to get into the matrix. As long as hydrogen ions stay within the matrix, oxidation of the fuel will happen continuously.
In the presence of an uncoupler, only phosphorylations are arrested, but oxidation happens continuously. In the presence of oligomycin, which inhibits the Fo component of the ATP synthase complex, there is no way hydrogen ions can get into the matrix. Without hydrogen ions, electron transport will stop.
When electron transport stops, oxidation of fuel will also stop. The only difference between uncouplers and oligomycin is that in oligomycin, not only phosphorylations but also oxidation are arrested.
Atractyloside
It inhibits the ATP-ADP translocator of mitochondrial membranes. ATP ADP translocator takes an ADP and gives out ATP, which is inhibited by Atractyloside.
Important One Liners
- The ETC Complex with copper is Complex IV
- Mobile complexes are Q and cytochrome C
- The complex in ETC without Heme is Q
- The final acceptor of electrons in ETC is Oxygen
- Complex II is Succinate dehydrogenase
Important MCQ's
Q1. According to Mitchelle's chemiosmotic theory, which of the following is true?
a. They are arranged in a decreasing order of redox potential
b. They are arranged in a decreasing order of ability to get reduced
c. They are arranged in a decreasing order of energy level
d. They are randomly arranged
Ans. The correct answer is (c )
Q2. The number of H+ translocated, when FADH2 enters ETC:
a.10
b. 6
C.3
d. 2
Ans. The correct answer is (b)
Q3. Antimycin inhibits the electron transport at which level:
a. Complex I to Q
b. Complex II to Q
C. Complex III to C
d. Complex IV to Oxygen
Ans. The correct answer is (c )
Q4. An obese woman, who is desperate to lose weight, takes an online weight reduction pill and ends up in hyperthermia. A paramedic checks the content of the pill and finds valinomycin as one of the components. Mechanism of action of valinomycin is
a. It is an uncoupler
b. It inhibits ATP synthase
c. It inhibits ATP/ADP translocator
d. It inhibits electron transport between Complex I and Q
Ans. The correct answer is (a)
Q5. A compound was added to isolated mitochondria in a medium containing succinate, fumarate, ADP, and Pi, and oxidative phosphorylation was decreased. Name the compound.
a. Oligomycin
b. 2,4-dinitrophenol
c. Antimycin
d. Rotenone
Ans. The correct answer is (a)
Q6. Marilyn Monroe, the famous American model, actress, and singer, was suffering from insomnia. Her friend introduced her to a street drug "Lilly 33s.", which is amobarbital!! She died at the age of 36, following intake of a few "Lillies" !! Amobarbital acts by inhibiting electron transport from
a. Complex I to Q
b. Complex II to Q
c. Complex III to C
d. Complex IV to Oxygen
Ans. The correct answer is (a)
Q7. Inhibitor X acts on a transporter marked in the image. Inhibitor X is
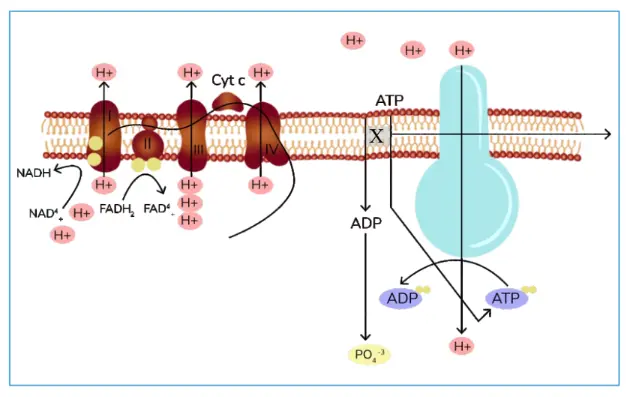
a. Hydrogen Sulphide
b. Oligomycin
c. 2,4 Dinitrophenol
d. Atractyloside
Ans. The correct answer is (D)
Download the PrepLadder app now to access high-yield content with 24-hour Free Trial. Explore premium study resources like Video Lectures, digital notes, QBank, and Mock Tests for seamless exam preparation. Start your NEET PG online coaching journey with PrepLadder.

PrepLadder
Access all the necessary resources you need to succeed in your competitive exam preparation. Stay informed with the latest news and updates on the upcoming exam, enhance your exam preparation, and transform your dreams into a reality!
Navigate Quickly
Electron Transport Chain
Oxidative Phosphorylation
Malate Shuttle
Glycerol Phosphate Shuttle
Difference between the malate shuttle and the glycerol phosphate shuttle
Complexes of the electron transport chain
There are 5 stationary complexes and 2 mobile complexes:
Q. Why is 2.5 ATP for NADH and 1.5 for FADH2? Why does this discrepancy occur?
Inhibitors of ETC
Uncoupler
Effects of uncouples
Definition of uncouplers
Types of Uncouplers
Important One Liners
Important MCQ's
Top searching words
The most popular search terms used by aspirants
- NEET PG Biochemistry
- NEET PG Biochemistry Preparation
PrepLadder Version X for NEET PG
Avail 24-Hr Free Trial